
Kombucha Defense Mechanisms
Kombucha is a living symbiosis of bacteria and yeast that has been brewed in homes and shared with others for hundreds if not thousands of years. A living culture with such a long history must have some way of protecting itself from causing harm to those who care for it (i.e. homebrewers) or surely it would have been ditched generations ago.
While our ancestors simply had to trust their gut, modern science is discovering the important role fermented foods have played in our culinary culture since recorded history (if not longer). The Human Microbiome Project has just begun to illuminate our understanding of the relationship between gut bacteria and health, but some of the preliminary findings are confirming what our ancestors instinctively knew: As Bacteriosapiens, fermented foods provide us with the regular influx of healthy, living bacteria our bodies need in order to boost proper functioning. The Kombucha culture evolved a few different defense mechanisms to protect itself from invasion from harmful microorganisms – low pH, ethanol and the SCOBY itself are all means that ensure the longevity of the culture. Let’s take a look at pH and the role it plays in protecting the culture.
pH & Kombucha
As you recall from the Top 5 Signs of Healthy Kombucha Brew, pH plays an important role in protecting the SCOBY from microbial invaders. Kind of like a chemical force field, the low pH creates a highly acidic environment in which our native bacteria and yeast thrive but simultaneously inhibits the growth of disruptive foreign & potentially harmful microorganisms.
pH was first conceived by Danish chemist Søren Peder Lauritz Sørensen at the Carlsberg Laboratory in 1909. Carlsberg Lab was set up by the Danish beer brewing company to advance biochemical knowledge, especially as applied to brewing beer. While studying proteins, Sørensen devised the pH scale as a means to express the concentration of hydrogen ions present in a solution.
While there are conflicting explanations for the definition, the most commonly accepted answer is that “p = potential” and the “H = hydrogen.” pH is a measurement of the acidity or alkalinity of a solution. When a substance dissolves in water, it produces charged molecules known as ions. Acidic water contains extra hydrogen ions (H+) and basic (alkaline) water contains extra hydroxyl (OH-) ions. As we can see on the chart below, the relationship from one pH level to the next is exponential.
The pH scale ranges from 0-14 with readings in the 0-7 range termed acid and readings in the 7-14 range termed alkaline. 7 is considered neutral and the ideal pH for our blood is just above 7. Without going into too many details, there is an important correlation between pH and health. While Kombucha tests on the acid side, much like lemon juice or apple cider vinegar, when it hits the digestive system it creates an alkaline ash.
******
******
The symbiosis of Kombucha cultures occurs between the yeast and bacteria, organisms which have evolved to work together via a system of balanced competition, with each contributing to the health and life cycle of the other. Acetobacter/Gluconacetobacter, the dominant type of bacteria in Kombucha, creates acetic acid – one of the most healthy acids and a clue to Kombucha’s low pH.
S. cerevisiae under an electron microscope. (source: Chemistryland.com)
Most have heard of the health benefits of acetic acid in the form of consuming small amounts of vinegar, which is typically a 4-7% acetic acid solution, whereas Kombucha checks in at a much more palatable ~1% acetic acid, often much lower in commercial versions.
On the pH scale, white distilled vinegar tests around 2.4 pH while a properly brewed batch of Kombucha may fall anywhere from 2.5 – 3.5. With this knowledge we can then infer (and taste!) that the lower the pH, the tarter the Kombucha. Obviously then, the obverse is also true – the higher the pH, the sweeter the Kombucha.
However, pH does NOT indicate that the Kombucha has finished the fermentation process. It confirms that the brew is protected from microorganisms. The pH should drop to the correct range of 3.5 and lower within the first few days of brewing. We use our most sensitive tool – our tongues – to decide when the appropriate amount of sugar has been converted.
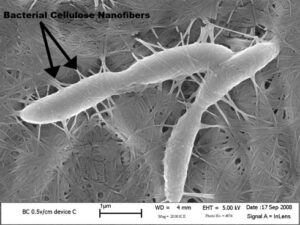
Acetobacter xylinium create nano fibers that knit together creating the SCOBY.
So while liquids with high concentrations of acetic acid have a low pH, they are correlated but not directly related. For example, apple cider vinegar (5% acetic acid) from my pantry tests as 2.9 pH while my flavored homebrew Kombucha (~1% acetic acid) comes in at 2.7 pH. Yet the ACV is undrinkably sour and disgusting while the Pink Lemonade Kombucha is dry, tart and delicious. By tasting and testing, one can train the palate to discern the subtle differences in flavor and match them to the pH value, especially when tracked along with brewing time.
According to the FDA’s guidelines for compliance, foods with a pH of 4.6 and lower have been deemed safe for sale without needing further preservatives: “When the pH of a food is 4.6 or below, spores of C. botulinum will not germinate and grow.” This means that not only is your KT safe from invasion by harmful microbes, but so are you!
 Kombucha Mamma Sez: While incorporating a variety of ferments in your diet is key, IMHO Kombucha is the queen of ferments because it is made from tea which has already been proven to have a host of health benefits.
Kombucha Mamma Sez: While incorporating a variety of ferments in your diet is key, IMHO Kombucha is the queen of ferments because it is made from tea which has already been proven to have a host of health benefits.
The Ideal pH for Kombucha is 3.5 – 2.5
Researchers in Brazil found that Kombucha’s antimicrobial activities protected against E. coli, Salmonella typhi & M. canis and were found to be most effective after 28 days of fermentation (perfect for Continuous Brewers!). Their research also revealed the cyclical nature of the symbiosis. After about 14 days of steadily declining pH, the activity shifted and the pH gradually rose and peaked again at 21 days, then shifted lower again at 28 days (see graph below).  These peaks and valleys indicate the ebb and flow of the symbiotic process of Kombucha.
These peaks and valleys indicate the ebb and flow of the symbiotic process of Kombucha.
First the yeast consume the sugar and produce CO2 & ethanol, then the bacteria consume the ethanol and produce healthy acids. The process repeats itself as the bacteria and yeast feed upon each other’s waste products. Adding sugar in the bottling stage reactivates this process and yields a different flavor than a freshly decanted Kombucha tea. Priming sugar is either a pinch of sugar added to the bottle or comes from the fruit or other flavoring agents. When added in the secondary stage, in an air tight environment, it reactivates the yeast which releases CO2 into the liquid. This is how we boost the natural carbonation that so many Kombucha drinkers love.
This low pH environment also means that Kombucha seldom “goes bad” in the way that we normally think of food spoilage. As Sandor Katz has said, fermentation is one of nature’s methods of preservation. The low pH prevents organisms responsible for spoilage from surviving. The ferment remains active in both an anaerobic environment (without oxygen, i.e. in the bottle) and at colder temps (i.e. in the fridge), just at a much slower pace.
Good Sanitary Practices are Key! As with all food preparation, it is important that sanitary practices are observed in order to prevent illness. Here are some tips for safely brewing Kombucha at home.
How to Test Your Kombucha pH
The easiest way to test the pH of your Kombucha is with pH strips or with a pH meter. If using pH strips, be sure they are in the correct range for your Kombucha (0-6). The strips are an easy, disposable way to check that the brew has reached the correct level of acidification. The meter offers greater precision and uses.
![]() Kombucha Mamma Sez: You don’t have to test the pH of your Kombucha unless you want to. However, these modern testing tools can provide peace of mind that the brew is progressing correctly as well as an interesting lesson for young and old – plus the meter is a easy way to track other ferments or your own body’s pH if you like!
Kombucha Mamma Sez: You don’t have to test the pH of your Kombucha unless you want to. However, these modern testing tools can provide peace of mind that the brew is progressing correctly as well as an interesting lesson for young and old – plus the meter is a easy way to track other ferments or your own body’s pH if you like!
 pH Strips
pH Strips
- Dip the pH strip in the Kombucha tea.
- Immediately compare with the color coded guide.
- The closest color match indicates the pH level.
pH Meter
- Calibrate the pH meter with the included buffer solution packet. Use the included screwdriver to manually adjust the setting to 7.0
- Rinse off buffer solution with filtered water.
- Place the pH meter in a small dish of Kombucha.
- It may take 10-30 seconds for the meter to settle on the correct reading.
- Use a small piece of damp sponge at the bottom of the black cap to keep the electrode moist.
Tasting your Kombucha and testing the pH will help you recognize the different stages of the brewing cycle. Once they are familiar, you will easily be able to detect where you are at in the process with just a sip or two!
 pH Strips
pH Strips
Alex
March 21, 2025 at 8:35 amHello, Hannah! Question about secondary fermentation in bottles (made with apple pieces and cinnamon). Everything turned out delicious, but when you don’t drink the first bottle right away, after some time the kambucha takes on the appearance of a gelatinous liquid (like aloe juice), although it is stored in the refrigerator. What could be the reason for this, and is it normal? Thank you!
kombuchaoffice@gmail.com
March 27, 2025 at 7:59 pmThis is due to the pectin in the fruit. To prevent the gelatinous texture, infuse the flavor for 24-48 hours, then strain and bottle. Hope this helps!
Jose Alberto
August 1, 2024 at 3:33 pmPuedo almacenar mis botellas de kombucha una vez cerradas a temperatura ambiente sin necesidad de frigorífico??
kombuchaoffice@gmail.com
August 10, 2024 at 12:16 pmYes, you can store your kombucha bottles at room temperature after closing them, but keep in mind that the fermentation process will continue, which can increase carbonation and change the flavor. To slow down the fermentation and maintain the taste, it’s best to refrigerate the bottles.
Sí, puedes almacenar tus botellas de kombucha a temperatura ambiente después de cerrarlas, pero ten en cuenta que el proceso de fermentación continuará, lo que puede aumentar la carbonatación y cambiar el sabor. Para ralentizar la fermentación y mantener el sabor, es mejor refrigerar las botellas.
Daniel
April 19, 2022 at 4:01 pmHi there Hannah! Not wanting to gush over your inspiration too much (here), let me just say your book and resources have helped me lots – many thanks to you and Alex! I was wondering if maybe you could tell me, if i’m measuring the acetic acid present in my brew directly (besides pH), what values should i be looking for ideally? With best wishes from Zurich, Switzerland <3
Hannah Crum
May 13, 2022 at 10:58 amThanks for your kind words, Daniel! We have been seeing TA (Total or Titratable Acidity) for Kombucha between .3-1.5% – in fact, we are launching a new, simple to use home testing product for TA. Check it out!
Grant
November 15, 2020 at 12:29 pmHi,
Quick question: my PH seems to have risen during F2 from 2.3 (… end of F1) to now 3.5 after 7 days …. this seems counterintuitive – is this a known problem?
Hannah Crum
December 4, 2020 at 1:24 pmAre you using pH paper or a meter?
Clifford Stork
February 22, 2020 at 12:14 pmIf you get a butyric smelling kombucha, your book says to dump the batch and start over. I’m doing a continuous brew in an almost 3 gallon container. I don’t mind restarting the batch but your book doesn’t specify if I need to dump the scoby too? Or can I restart the batch with that scoby and starter liquid from my other continuous brew which tastes fine? Thanks in advance for any input!
Hannah Crum
December 7, 2023 at 2:08 pmJust the brew – the SCOBY is probably fine. Could be a contamination from water, tea type, sugar type, etc.
Gregor
October 5, 2019 at 12:11 pmThanks for all your invaluable info, Hannah! I have brewed Kombucha in four one-gallon jars and a two-gallon vat for years now, and find my batches taste optimal in about eight days. (Yield about 5-1/2 gallons every eight days.) I regularly peel back my healthy monster SCOBYs so they don’t get too huge, and generally leave the yeast dregs in the bottom of the jar for the next batch. From this article it seems I should reserve some liquid from the top of the fermenter instead of the bottom dregs, even though it has worked well so far. I do not add flavorings or add priming sugar at bottling, but my carbonation is reliably good (occasionally a tad over-frisky, but few gushers).
Even a 9-day fermentation proves too tart using my cultures, but the diagram above reads like the pH will rise for two weeks after the first week’s fast fermentation, then drop back down over the fourth week, hitting the desired 2.5-3.5 pH around Day 28. Do you think I should devote one of my gallon jars to this much longer process? What benefit is there to a long fermentation that I’m missing with my present method? Thanks!
Hannah Crum
November 4, 2019 at 8:57 amThe reason its too tart after 9 days is because of the yeastie dregs – switching to the bacteria rich starter liquid from the top will balance that out. Longer fermentation yields different acids. Of course, if you like your process and flavor the way it is, you could stick with it.
Chris
May 23, 2019 at 7:21 pmMy combucha has been fermenting for 2 weeks. A nice scoby has formed on the top. My problem is the ph of the combucha is 7.0. Not safe for bacteria. The scoby I got on Amazon was brown with strings. I would try again but have no acidic starter liquid.
Hannah Crum
May 24, 2019 at 9:00 amSounds like your pH strips or meter is not accurate. Check out our testing tools here –> https://store.kombuchakamp.com/kombucha-testing-tools/
Patsy
May 7, 2018 at 9:42 pmHannah, I’ve been brewing Kombucha for several years and it usually takes 7 to 10 days but recently it finishes at about 3 to 4 days. The ph is 3. It taste good, not sweet and not vinegary. Is this ok or do I need to be brewing longer. I’m concerned that I might lose the health benefits of such a short brew. I always do a second ferment for carbonation and it never takes more than 3 days unless I put fruit or juice in it and then it carbonates overnight.
Hannah Crum
June 28, 2019 at 3:19 pmIf you are doing Continuous Brew, this is a sign that its time to clean the vessel. If you are in batch brew, the first question is where are you taking the starter liquid from – the top or the bottom? Our guess would be the bottom which is why its getting sour so quickly as the yeast and bacteria are being thrown out of balance. A sure sign the yeast are dominating the bacteria is that it sours more quickly and there is less SCOBY growth. Start taking your liquid from the top of the vessel rather than just leaving dregs at the bottom and it will gradually rebalance in a few cycles.
Jennifer O Castles
February 5, 2018 at 1:34 pmThank you Hannah Crum. This has been very informative and given me a new perpective on Kombucha brewing. I love it, i infuse the tea with herbs and fruits around the 10th day for 2nd fermentation. I have shared my scoby and it has reached far and wide. I dont profess to be knowledgeable in all aspects but i shared my recipe and made myself available to them 24/7 if they needed troubleshooting or the anxious first time brewer. Its all about using ones common sense, i encourage them to taste their brew at day 8, a great indicator of brew fermentation of taste and flavour and to let it continue brewing if its too sweet. This journey has done wonders for me, i have type 2 diabetes and have been detoxing for my heath and wellness. My blood pH has since dropped 2 since i started, ive since shared a bottle with my doctor who was very impressed.
Deb
August 4, 2023 at 5:26 pmHi Jennifer, I am very interested in learning how to brew with stevia since I have diabetes 2. I understand I need sugar to start fermentation but how long till the sugar is converted till co2 and alcohol.
Deb
Simon
January 22, 2018 at 9:15 amHello. Can you please expand more about: « – a Brix measurement ». I do have a refractometer low range to measure sugar level in juice. Does this instrument works for Brix measurement?
Hannah Crum
June 14, 2019 at 7:53 pmThe refractometer measures Brix – so that device will work!
Alan
August 9, 2017 at 11:44 amI don’t like my kombucha too tart so stop the first fermentation around PH 3.3. The problem is I then add fruit for the second fermentation and this raises to PH to above 3.6 and it stays there once the second fermentation is finished.
Does this mean my drink is no longer technically kombucha?
Hannah Crum
July 7, 2018 at 9:19 amNot at all! As long as it tastes delicious and it is below 4.6 then it is safe to drink!
J. Belliveau
May 25, 2017 at 6:09 amHi, I’m a first-time brewer….. I bottled my first batch 2 days ago and placed the bottles in the refrigerator immediately. Today I read that I should have placed the bottles in the cupboard for 3 or 4 days before refrigerating. Did I ruin the batch….is there a fix for this?
Hannah Crum
May 14, 2018 at 1:37 pmTake them out of the fridge and let them stand at room temp for a day or two until the flavor infuses & fizz develops. Need more carbonation tips? https://www.kombuchakamp.com/kombucha-carbonation-for-beginners
Carol
May 22, 2017 at 1:48 pmIf my continuous brew is less than two months old and the KT is tasting vinegary within 2-3 days with a pH of 3.0, should I still clean out the brewer and start with fresh nutrient brew? The scoby is only 5 months old.
Hannah Crum
May 14, 2018 at 1:45 pmYes – anytime the brew ferments too quickly, that’s a sign that its time to clean out the excess yeast and discard any excess SCOBY. We’d recommend keeping 10-16oz of SCOBY in the vessel at all times, the rest may be disposed of or used like this –> https://www.kombuchakamp.com/kombucha-scoby-cultures-top-5-other-uses
Andrea
May 17, 2017 at 5:01 pmWhat if my ph is at a 4 (used the ph strips)? It still tastes sweet at 7 days. Do I need to start over or give it another week to ferment?
Hannah Crum
May 3, 2018 at 5:40 pmCould use more time. Fermentation is temperature dependent. If its too cool or taking too long, consider adding a heater.
Joe
May 12, 2017 at 4:04 pmThis is my second brew. The first brew went well under the same conditions. This second brew dropped to a PH of 3.3 after 3 days. It is now day 11 and the PH is sitting at 3.9. The taste is still a little sweet, and I seem to have a brain fog shortly after ingesting. This has never happened to me after drinking kombucha. No signs of mold, and a new Scoby is growing. Any advice? Should I throw this batch away?
Hannah Crum
May 3, 2018 at 5:37 pmIf you run into a “stuck” fermentation where the pH isn’t lowering, then it might need more oxygen. Try stirring it with a spoon (don’t worry, you won’t hurt the SCOBY) and see if it doesn’t start to drop again in a few days.
RT
April 16, 2017 at 4:50 pmHave maggots growing on top of scobie and dead maggots in liquid. Very small maggots.
Help!
Hannah Crum
April 17, 2017 at 6:23 pmCheck out our post on fruit fly control –> https://www.kombuchakamp.com/fruit-fly-trap-video-quick-tip
Austin
April 6, 2016 at 6:25 pmThe p in pH stands for -log (base 10). A pH of 3.0 means there are 10^-3 moles per liter of H+ ions.
Maria
August 15, 2015 at 12:00 amI didn’t know we had to test the ph range. I just drank about 8oz of 2fermented kombucha and didn’t test it. Is this a health hazard?
Hannah Crum
September 2, 2015 at 8:15 ampH testing is for troubleshooting. Provided there is no mold on the cultures then you are good!
Mike
August 6, 2015 at 11:31 amI read about a business that does “dry” brewing that has a very low sugar content in it’s but. How is this done?
Hannah Crum
August 11, 2015 at 7:30 amReducing the amount of sugar also reduces the flavor. You can try it by using different amounts of sugar. Experiment to find the qty that yields good flavor and also encourages SCOBY growth and proper fermentation.
sherri smoot
August 4, 2015 at 11:35 amI’m new to continuous brewing and I’m nervous because my kombucha is always very vinegary. I’ve tried draining some of it off and adding black sweetened tea to neutralize it. But it seems that only a day or two later it’s very vinegary again. So it’s either sweet and tastes like tea or vinegary. What am I doing wrong? Where do I find that happy medium? It tastes too vinegary to drink.
Hannah Crum
August 11, 2015 at 7:34 amIf it sours too quickly, that is a sign it is time to clean out the vessel. Refer to the Complete Handbook – Chp 5 & 8 or the EZ UPkeep videos for full instructions on how to do that.
nancy
July 6, 2015 at 10:13 amCan alkaline water be used to make kombucha? I have a kangen water filter. It has several different pH options…9.5,9.0,8.5,7.0,6.0 and 2.5. Which would be good to make my sweet tea with?
Hannah Crum
July 9, 2015 at 7:18 pmSince Kombucha is an acidic food, it would be best to start with water that has a lower pH such as 6.0
Stephanie
May 19, 2015 at 9:53 pmWe are new to making Kombucha and are on our 4th batch, starting from a dehydrated scoby. So far the brew has been almost too tart for our liking and I don’t know how to address that. Today was day 5 for this current batch. I tested the pH and it was 2.8. Should it be higher for it to taste less tart? How do I accomplish this? Thanks so much for your help!
Hannah Crum
May 21, 2015 at 2:42 pmWe don’t recommend using dehydrated cultures as they lack the starter liquid needed for optimum flavor and fermentation (see article here).
If you are using vinegar as your starter liquid, it may be dropping the pH too quickly which may cause the brew to be too tart, though pH alone will not be enough to determine if it is too sour – a Brix measurement or using your taste buds will offer that additional information.
Lisa
May 5, 2015 at 6:47 pmI am in the process of brewing my first batch of Kombucha. I am on day 8, and have a healthy scoby, but a pH level of 5-6. I am not clear when the lower pH happens..after bottling! Is there a correlTion between pH level and when to bottle? Thx.
Hannah Crum
May 11, 2015 at 6:56 amThe pH of Kombucha drops to 3.5 or lower within the first 3 days if all of the conditions are correct. If your pH is that high, then something is off in the brew. First, confirm the pH strips you are using can tolerate the lower range of Kombucha (0-6) or consider purchasing a meter (make sure it is properly calibrated). Then test again. If the pH is still at that level, then we would not recommend consuming it. The correlation between pH and bottling is not accurate unless other metrics such as Brix and the brewing conditions are also factored into the equation.
wilma mountain
March 25, 2015 at 4:54 amI have been brewing booch 4 months i live in minnesota where the weather is strange ,a couple batches got mold.So starting out fresh .Is it ok to put on top of fridge /out of direct sun.I have 4 vessels going .LOVE THE booch ,gave scoby away to random person whom showed interest.
Hannah Crum
April 8, 2015 at 11:27 pmYes – on top of the fridge out of direct sunlight is a great place for Kombucha. The warm air from the fridge exhaust can help keep it warm. Kombucha inspires sharing!
Rebecca
February 13, 2014 at 10:20 amI just started brewing kombucha and am doing the continuous brew. I started testing the ph on day 8, and it was 3.5, and now on day 12 it is still 3.5. I have maintained the temperature between 78-82 and am wondering if this is normal that it would take this long? Is there anything I should be doing? It still tastes really sweet to me. Thanks!
Hannah Crum
February 14, 2014 at 3:06 pmAs the post states, pH is not an indicator of doneness but rather an indicator that your brew is protected. Taste is how we determine when our booch is ready to enjoy!
Miles Exner
February 4, 2014 at 2:12 pmOk, did some research and looked up the translation of kun and bu (the pinyin versions). “Giant fish cloth that covers” cha “tea”. So it seems to be referring to the appearance.
Miles Exner
February 4, 2014 at 10:28 amHow did kombucha evolve? In what environment? I doubt tea water is a natural occurrence. Was it bred into that environment? Kombu is Chinese for a type of seaweed, but I never hear any mention of it. It may have another meaning, but cha must likely means tea. Was kombu originally used? If you find any information regarding this, will you please email me. Thanks!
Hannah Crum
February 6, 2014 at 7:32 amYou can read all about it in our forthcoming book (Spring 2015)